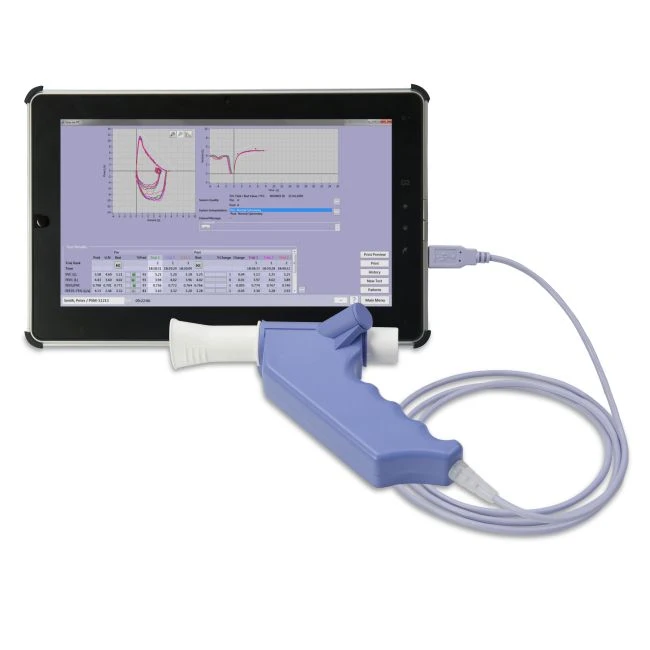
Quote Information
Commercial Procurement Made Easier
New healthcare facility construction, expansion or remodel - we’ve created a streamlined approach to healthcare supply chain for our following customers:
- Medical facilities (Hospitals, Physician Offices, Long Term Care facilities, etc.)
- GPO members (Vizient, Premier, Intalere, Healthrust, ROi)
- Government Agencies (VA, DoD)
- Educational Institutions (Universities, Schools, etc.)
- other bulk buyers
How to Request a Quote
Simply search for your desired product(s), and click ‘Chat with Us for GPO Pricing!’ to connect with one of our product specialists. They will note your contact information and any comments/questions about the item or an overall quote, and submit it to an account manager. One of our dedicated account managers will reach out to you with your pricing and to discuss your logistics needs within 12 hours.
CME Makes It Easy
- 2 million+ products, from over 2000 manufacturers.
- Contract pricing with all the major GPOs, and volume discounts for bulk orders.
- We save you time and money by being your most competitive source for all items in your PO/quote, and we’ll even locate those products we don’t carry at the best price.
- Streamlined, on-time delivery with turn-key white glove service, scheduled for when its best for you.
Quote to Delivery. One Team. One Partner. CME Makes It Easy.