| Discontinued | No |
|---|
| Additional Specifications: | |
|---|
| Feature | Specification* |
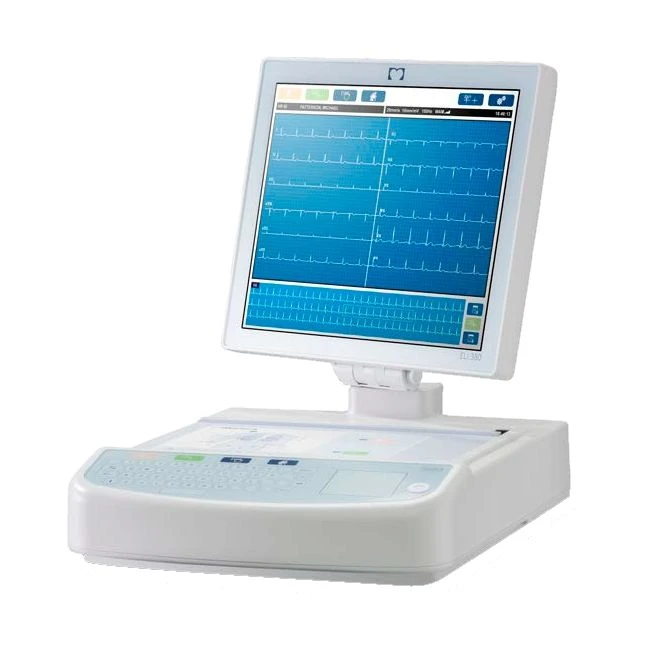
| Instrument Type | Multi-lead resting electrocardiograph |
| Input Channels | Simultaneous acquisition of all 12 or 15 leads |
| Standard Leads Acquired | I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6 |
| 12 Lead Alternate Lead Groups | Up to three additional groups can be labeled using any combination of 6 precordial leads with
V1, V2, V3, V4, V5, V6, V7, V8, V9, V3R, V4R, V5R, V6R, and V7R lead labels |
| 15 Lead Groups | Three lead groups are available and can be labeled using any combination of 3 leads with
V1, V2, V3, V4, V5, V6, V7, V8, V9, V3R, V4R, V5R, V6R, and V7R lead labels |
| Waveform Display | ERGO: Backlit, 17” color touchscreen display, 180° rotation and 120° tilt
Standard: Backlit, 17” color LCD with 1280 x 1024 resolution, 120° tilt |
Input Impedance
Input Dynamic Range
Electrode Offset Tolerance
Common Mode Rejection | Meets or exceeds the requirements of ANSI/AAMI/IEC 60601-2-25 |
Patient Leakage Current
Chassis Leakage Current | Meets or exceeds the requirements of ANSI/AAMI ES 60601-1 |
| Digital Sampling Rate | 40,000 samples/s/channel used for pacemaker spike detection;
1,000 samples/s/channel used for recording and analysis |
| Standout Clinical Features | Best 10 automatic capture of the 10 seconds of data with the least amount of noise from the last 5 minutes
of full disclosure; Alternate lead placement selection with default for pediatric, right-sided, posterior and any
combination of user-defined precordial lead labeling. |
| Optional Functions | Connectivity with bidirectional communication; SECUR-it; 15-lead acquisition; supports bar code scanners
with 39, 128 and 2D capabilities; AM12™, AM12M, or AM15E acquisition module, equivalent in size and weight
to a traditional patient cable |
| Paper | Smart (210 x 280 mm), perforated Z-fold thermal cued paper with full grid; 250 sheets stored in paper tray |
| Thermal Printer | Computer-controlled dot array; 1 dot/ms horizontal, 8 dots/mm vertical |
| Thermal Printer Speeds | 5, 10, 25 or 50 mm/s |
| Gain Settings | 5, 10 or 20 mm/mV |
| Report Print Formats | Standard or Cabrera: 3+1, 3+3, 6, 6+6 or 12 channel |
| Rhythm Print Formats | 3, 6, 8 or 12 channel with configurable lead groups |
| Keyboard | Glass keyboard with alphanumeric keys, soft-key menu, dedicated function keys, and touchpad pointing device |
| Frequency Response | 0.05 to 300 Hz |
| Filters | High-performance baseline filter; AC interference filter 50/60 Hz; low-pass filters 40 Hz, 150 Hz, or 300 Hz |
| A/D Conversion | 20 bits (1.17 microvolt LSB) |
| Device Classification | Class I, Type CF defibrillation-proof applied parts |
| ECG Storage | Internal storage up to 500 ECGs |
| Information Exchange | Requires ELI Link software version 4.2.0 or greater |
| SECUR-IT | Requires software version 1.2.0 or greater and ELI Link software version 4.4.0 or greater |
| AM12M Compatibility | For use with Quickels Electrode Suction Pump Decapus III or greater |
| Weight | ERGO: 27 lbs (12 kg) including one battery (without paper) |
| Dimensions | 15.5 (width) x 20 (depth) x 5.5 in (height) (39 x 51 x 14 cm) |
| Power Requirements | Universal AC power supply (100-240 VAC at 50/60 Hz) 110 VA; internal rechargeable lithium-ion battery with
support for a second optional battery |